Transcriptional Events in Remyelination by Human Oligodendrocyte Progenitors
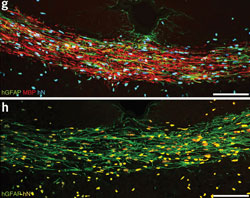
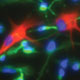
Sections of a CD140a+ cell–engrafted shiverer callosum at 12 weeks, immunostained for MBP, human GFAP and human nuclear antigen (showing robust production of hGFAP+ astrocytes as well as MBP+ oligodendrocytes).
This project focuses on defining the gene expression changes associated with in vivo remyelination by human oligodendrocyte progenitor cells, using our novel human glial chimeric brain model. By way of background, little information is available as to the changes in gene expression or dominant pathway activation associated with the mobilization, oligodendrocyte fate commitment, or myelination by human oligodendrocyte progenitor cells during the process remyelination. To accomplish this goal, and to do so using human cells in vivo, we have established human glial chimeras by neonatal implantation of human glial progenitor cells into the forebrains of neonatal immunodeficient mice; in these mice, the human progenitor pool outcompetes their mouse counterparts, resulting in the eventual replacement of the mouse glial progenitors by their human counterparts. By subjecting these mice to cuprizone demyelination, and then following the resultant compensatory remyelination by the human progenitor cells, we can track remyelination by human cells in vivo, both by direct imaging, and by transcriptional analysis of sorted human progenitors. For the latter purpose, the remyelinating human progenitor are being sorted from their host brains by surface antigen-based FACS, and their transcriptional profiles assessed by both microarray and RNAseq. These data will provide us the transcriptional events associated specifically with progenitor mobilization and remyelination, and as such should provide new targets for accelerating and potentiating this process following acute demyelinating episodes.