Use of hiPSC and hESC-derived Neural Progenitors for Modeling Neuropsychiatric Disease
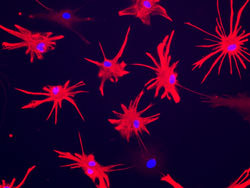
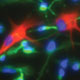
Glial form and function are extraordinarily divergent with evolution, and human astrocytes are virtually unique in their pleomorphism and fiber complexity. In particular, human astrocytes are larger and more structurally complex than rodent glia, and coordinate the actions of vastly more synapses within their geographic domains. To assess the relative contributions of glial cells to the species-specific aspects of human cognition, we had previously engrafted neonatal mice with human glial progenitor cells (GPCs), to establish brains chimeric for human astrocytes. In collaboration with Dr. Nedergaard’s lab, we then assessed these glial chimeric mice functionally and behaviorally, and found that they exhibited substantially enhanced activity-dependent plasticity and learning. This work established the potential of using human glial chimeras to assess human-specific aspects of the contributions of astrocytes to cognition, and to its disorders.
Such astrocytic involvement in human cognitive disorders has never been studied, and yet its role may be profound. As a case in point, a number of conditions, especially several neuropsychiatric disorders, are specific to humans. Yet while human neuronal cytoarchitecture is not very different from that of primates, astrocytic pleomorphism exhibits a quantal leap with human evolution, concurrent with the appearance of a number of neuropsychiatric conditions that appear unique to humans. In particular, given the correlation between the appearance in humans of neuropsychiatric disorders such as schizophrenia and bipolar disorder, with that of uniquely human astrocytic morphologies and synaptic modulation, we are pursuing the specific possibility that human glial pathology contributes to the pathogenesis of schizophrenia.
To test this hypothesis, we have established human glial chimeric mice with glial progenitor cells derived from iPS cells themselves derived from patients with juvenile-onset schizophrenia, a category typically associated with a set of described mutations and copy number variants. By this means, we have generated human glial chimeric mice whose glia have been largely replaced by human glial progenitors and their derived astrocytes, in which the selective effects of schizophrenia-derived glia, on both cortical neuroanatomy and behavior may be assessed, relative to those derived from normal patient controls. This is a collaborative project with Maiken Nedergaard’s lab, which is responsible for the physiological and behavioral assessment of these animals, after the generation of the subject cells and chimeric animals by our group, which is also responsible for assessing the neuroanatomical and transcriptional concomitants of schizophrenia-derived human glial chimerization. Our hope is that we may define new glial targets for the treatment of schizophrenia, a disease whose treatment strategies have hitherto largely been limited to the suppression of dopaminergic signaling.