Division of Cell and Gene Therapy
The Goldman Laboratory
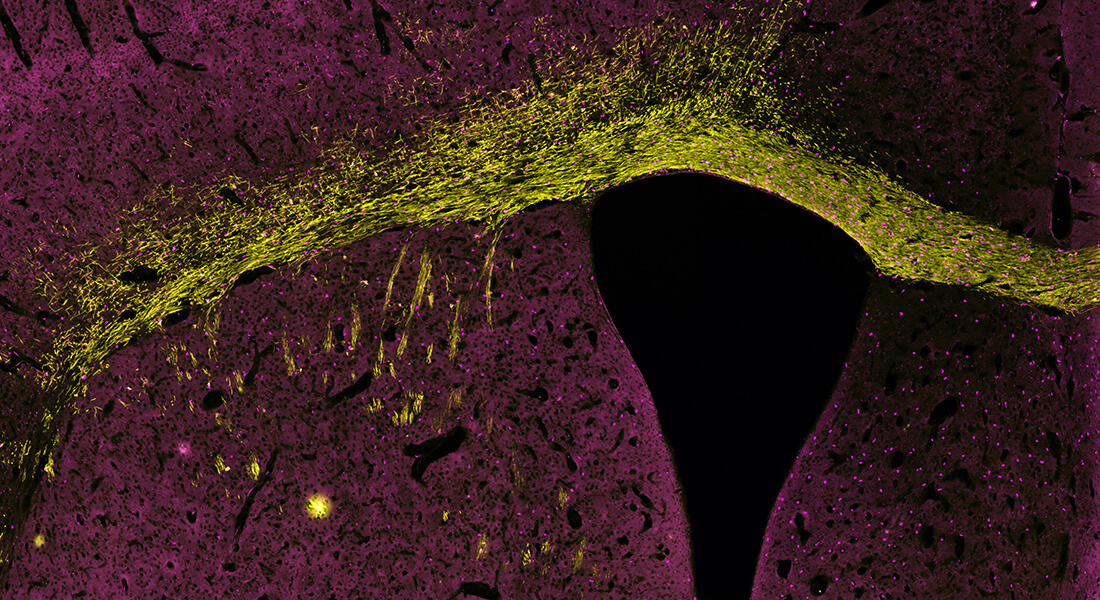
Our research
In particular, we are working on diseases that affect one type of cell, such as demyelinating diseases (using oligodendrocyte stem cells) and Huntington's disease (using spiny neuron replacement). At the same time, we are investigating gliomas and their origin in dysregulated glial progenitors. Our research covers a wide range of glial disorders, including multiple sclerosis, leukodystrophies and psychiatric disorders. We have a particular focus on cell transplantation in glial and myelin disorders and the activation of the brain's own cells to regenerate damaged neural circuits.
|
Issued US Patents (31 issued in US; 19 pending published PCTs listed in prosecution; list does not include >100 corresponding international patents) |
|
Method for Separating Cells Inventors: Steven Goldman and Hong Wu; Owner: Cornell Research Foundation, Inc. |
|
Method for Separating Cells Inventors: Steven Goldman and Hong Wu; Owner: Cornell Research Foundation, Inc. |
|
Discovery, Localization, Harvest and Propagation of an FGF2 and BDNF-Responsive Population of Neural and Neuronal Progenitor Cells in the Adult Human Forebrain |
|
Method of Inducing Neuronal production in the Brain and Spinal Cord |
|
Telomerase-Immortalized Neural Progenitor Cells |
|
Enriched Preparation of Human Fetal Multipotential Neural Stem Cells Inventors: Steven A. Goldman and Hideyuki Okano; Owner: Cornell Research Foundation, Inc. |
|
Non-Human Animals with Human-Glial Chimeric Brains Inventors: Steven Goldman and Martha S. Windrem; Owner: University of Rochester |
|
Enhancing Neurotrophin-Induced Neurogenesis by Endogenous Neural Progenitor Cells by Concurrent Overexpression of BDNF and an Inhibitor of a Pro-Gliogenic Bone Morphogenetic Protein Inventors: Steven Goldman, Eva Chmielnicki and Aris Economides; Owner: Cornell Research Foundation, Inc. |
|
Neuronal Progenitor Cells from Hippocampal Tissue and a Method for Isolating and Purifying Them Inventor: Steven Goldman; Owner: Cornell Research Foundation, Inc. |
|
Method of Inducing Neuronal Production in the Caudate Nucleus and Putamen Inventors: Steven Goldman and Abdellatif Benraiss; Owner: Cornell Research Foundation, Inc. |
|
Method of Inducing Neuronal Production in the Caudate Nucleus and Putamen Inventors: Steven Goldman and Abdellatif Benraiss; Owner: Cornell Research Foundation, Inc. |
|
Method of Inducing Neuronal Production in the Brain and Spinal Cord Inventors: Steven Goldman and Abdellatif Benraiss; Owner: Cornell Research Foundation, Inc. |
|
Purines are Self-Renewing signals for Neural Stem cells, and Purine Receptor Antagonists Promote Neuronal and Glial Differentiation Therefrom Inventors: Steven A. Goldman, Maiken Nedergaard, Jane Lin; Owner: Cornell Research Foundation, Inc. |
|
Purine Receptor Inhibition as a Therapeutic Strategy in Spinal Cord and Brain Inventors: Steven Goldman and Maiken Nedergaard; Owner: Cornell Research Foundation, Inc. |
|
Myelination of Congenitally Dysmyelinated Forebrains using Oligodendrocyte Progenitor Cells Inventors: Steven Goldman, Neeta Roy and Martha Windrem; Owner: Cornell Research Foundation, Inc. |
|
Myelination of Congenitally Dysmyelinated Forebrains Using Oligodendrocyte Progenitor Cells Inventors: Steven Goldman, Neeta Roy and Martha Windrem; Owner: University of Rochester |
|
Method for Isolating and Purifying Oligodendrocytes and Oligodendrocyte Progenitor Cells Inventors: Steven Goldman and Su Wang; Owner: Cornell Research Foundation, Inc. |
|
Enriched or Purified Population of Motor Neurons and its Preparation from a Population of Embryonic Stem Cells Inventors: Steven Goldman, Takahiro Nakano, Neeta Roy; Owner: Cornell Research Foundation, Inc. |
|
Regulatory Sequences that Direct Gene Expression to Spinal Motor Neurons and Uses Thereof Inventors: Steven Goldman and Takahiro Nakano; Owner: Cornell Research Foundation, Inc. |
|
Identification and Isolation of Multipotential Neural Progenitor Cells from the Subcortical White Matter of the Adult Human Brain Inventors: Steven Goldman and Neeta Roy; Owner: Cornell Research Foundation, Inc. |
|
Modulating the Production of Neurons and/or Oligodendrocytes from White Matter Progenitor Cells Inventors: Steven Goldman and Fraser Sim; Owner: Cornell Research Foundation, Inc. |
|
Treating Myelin Diseases With Optimized Cell Preparations Inventors: Steven Goldman and Fraser Sim; Owner: University of Rochester |
|
Non-Human Mammalian Model of a Human Degenerative Disorder, Uses Thereof, and Method for Treating Human Degenerative Disorders Inventor: Steven Goldman; Owner: University of Rochester |
|
Myelination of Congenitally Dysmyelinated Forebrains Using Oligodendrocyte Progenitor Cells Inventors: Steven Goldman, Neeta Roy and Martha Windrem; Owner: University of Rochester |
|
Non-Human Mammalian Model of a Human Degenerative Disorder, Uses Thereof, and Method for Treating Human Degenerative Disorders Inventor: Steven Goldman; Owner: University of Rochester |
|
Modulating the Production of Neurons and/or Oligodendrocytes from White Matter Progenitor Cells Inventors: Steven Goldman and Fraser Sim |
|
Induced Pluripotent Cell-Derived Oligodendrocyte Progenitor Cells for the Treatment of Myelin Disorders Inventors: Steven Goldman and Su Wang |
|
Induced Pluripotent Cell-Derived Oligodendrocyte Progenitor Cells for Treatment of Myelin Disorders Inventors: Steven Goldman and Su Wang |
|
A Human Glial Chimeric Model for Drug Candidate Assessment in Human Gliotrophic Viral Infections and Progressive Multifocal Encephalopathy (divisional filing) Inventors: Steven Goldman, Leonid Gorelick, Yoichi Kondo, and Martha Windrem; Owner: University of Rochester |
|
Treating Myelin Diseases With Optimized Cell Preparations Inventors: Steven Goldman and Fraser Sim; Owner: University of Rochester |
|
Non-Human Mammalian Model of a Human Degenerative Disorder, Uses Thereof, and Method for Treating Human Degenerative Disorder Inventor: Steven Goldman; Owner: University of Rochester |
|
Methods of Treating Neuropsychiatric Disorders Inventors: Steven Goldman and Maiken Nedergaard; Owner: University of Rochester |
Pending US Patent Applications (all have corresponding PCTs and international filings):
|
Human iPS Cell-derived Glial Progenitor Cells for the Patient-Specific Treatment of Myelin Disorders Inventors: Steven Goldman; Owner: University of Rochester |
|
Methods of Treating Schizophrenia and Other Neuropsychiatric Disorders Inventor: Steven Goldman; Owner: University of Rochester |
|
Methods of Treating or Inhibiting Onset of Huntington's Disease Inventor: Steven Goldman; Owner: University of Rochester |
|
Cell type-specific immunoprotected cell lines Inventors: Steven Goldman and Abdellatif Benraiss; Owner: University of Rochester |
|
Identification of transplanted human glial cells (by PET) Inventors: Steven Goldman, Gitte Moos Knudsen and Abdellatif Benraiss; Owners: University of Rochester and University of Copenhagen |
|
GPR17 Promoter-Based Targeting and Transduction of Glial Progenitor Cells Inventors: Steven Goldman, John Mariani and Abdellatif Benraiss; Owner: University of Rochester |
|
MicroRNA-Medicated Methods for Rejuvenating CNS Glial Populations Inventors: Steven Goldman and John Mariani; Owner: University of Rochester |
|
MicroRNA-Medicated Methods for Rejuvenating CNS Glial Populations Inventors: Steven Goldman and John Mariani; Owner: University of Rochester |
|
Humanized Chimeras for the Prospective Assessment of Cell Addition and Replacement Therapies Inventors: Ricardo Vieira and Steven Goldman; Owner: University of Rochester and the University of Copenhagen |
|
Gene Networks that Mediate Remyelination of the Human Brain Inventors: Steven Goldman and John Mariani; Owner: University of Rochester |
|
Methods Of Generating A Population Of Neurons From Human Glial Progenitor Cells And Genetic Constructs For Carrying Out Such Methods Inventors: Steven Goldman and Abdellatif Benraiss; Owner: University of Rochester |
|
Compositions and Methods for Delivery of Agents to Inner Ear Inventors: Maiken Nedergaard, Steven Goldman; Owner: University of Rochester |
|
Treatment of Age-Related White Matter Loss Inventors: Ricardo Vieira and Steven Goldman; Owner: University of Rochester and University of Copenhagen |
|
TCF7L2 Mediated Remyelination in the Brain Inventors: Steven Goldman, Abdellatif Benraiss, John Mariani; Owner: University of Rochester |
|
Treatment with Genetically Modified Cells, and Genetically Modified Cells Per Se, with Increased Competitive Advantage and/or Decreased Competitive Disadvantage Inventors: Steven Goldman, John Mariani; Owner: University of Rochester |
|
Inhibition of Repressors that Mediate Glial Aging as a Means of Rejuvenating CNS Glial Populations Inventors: Steven Goldman, John Mariani; Owner: University of Rochester |
|
BCL11A Mediation of Human Glial Progenitor Cell Rejuvenation from Senescence, Population Expansion, Oligodendrocyte Differentiation and Remyelination Inventors: Steven Goldman, John Mariani; Owner: University of Rochester |
|
Alleviating Adverse Effects of Age-Related White Matter Lose by Cell Replacement Therapy Inventors: Steven Goldman, John Mariani; Owner: University of Rochester |
|
Alleviating Adverse Effects of Age-Related White Matter Lose by Cell Replacement Therapy Inventors: Steven Goldman, John Mariani; Owner: University of Rochester |
|
A Humanized In Vivo Model of Competitive Cell Interactions for Predicting Therapeutic Efficacy of Cell Replacement Inventors: Steven Goldman, John Mariani; Owner: University of Rochester |
Incomplete list of current domestic and foreign filings; also does not include pre-PCT US filings or foreign patents issued.
The biology of human brain cells differs substantially from that of laboratory animals.
As a result, while the molecular data obtained from rodent models has been extraordinarily valuable in understanding basic cell and molecular biology of development, it has fallen short in identifying appropriate targets for therapeutic intervention; the predictive value of these models in modulating human brain function has proven marginal.
To provide a stronger basis for the lab’s translationally-oriented studies, we have thus established a molecular atlas of differential gene expression by human neural stem and progenitor cells of both the adult and fetal human brain. The generation of this atlas required the invention of several new techniques for phenotype-specific cell identification, FACS isolation and profiling, which comprise a coherent body of patents and papers spanning the past 15 years.
Our resultant genomic encyclopedia provides us a unique molecular database upon which to identify those transcriptional changes involved in both tumorigenesis and cell fate decisions. Going forward, we anticipate this database to be crucial over the next decade in essentially all of our major efforts: to induce neurogenesis and gliogenesis from endogenous progenitors; to abrogate tumorigenesis from endogenous progenitor cells; and to instruct differentiated cell fates from pluripotential stem cells.
Genes dysregulated in A2B5+ glioma cells at all stages of gliomagenesis.
This project focuses on defining the gene expression changes during anaplastic progression of isolated glioma tumor progenitor cells, derived and isolated during tumor progression from low grade gliomas through malignant gliomas and glioblastoma.
By first isolating defined phenotypes of gliomas-initiating tumor stem cells from different staged tumors, then normalizing the gene expression patterns of these cells to that of normal glial progenitor cells derived from normal tissue, and then comparing those genes differentially expressed by glioma stem cells at each stage of tumor progression, to one another, this project seeks to define those genes dysregulated at all stages of glial tumorigenesis.
Our intent in this regard is to identify those transcriptional patterns and predicted signaling pathways involved in glioma growth at all stages of tumor progression, yet not expressed by normal glial progenitors, as a means of identifying molecular targets for drug therapy unlikely to interfere with normal glial function.
Sections of a CD140a+ cell–engrafted shiverer callosum at 12 weeks, immunostained for MBP, human GFAP and human nuclear antigen (showing robust production of hGFAP+ astrocytes as well as MBP+ oligodendrocytes).
This project focuses on defining the gene expression changes associated with in vivo remyelination by human oligodendrocyte progenitor cells, using our novel human glial chimeric brain model.
By way of background, little information is available as to the changes in gene expression or dominant pathway activation associated with the mobilization, oligodendrocyte fate commitment, or myelination by human oligodendrocyte progenitor cells during the process remyelination.
To accomplish this goal, and to do so using human cells in vivo, we have established human glial chimeras by neonatal implantation of human glial progenitor cells into the forebrains of neonatal immunodeficient mice; in these mice, the human progenitor pool outcompetes their mouse counterparts, resulting in the eventual replacement of the mouse glial progenitors by their human counterparts.
By subjecting these mice to cuprizone demyelination, and then following the resultant compensatory remyelination by the human progenitor cells, we can track remyelination by human cells in vivo, both by direct imaging, and by transcriptional analysis of sorted human progenitors. For the latter purpose, the remyelinating human progenitor are being sorted from their host brains by surface antigen-based FACS, and their transcriptional profiles assessed by both microarray and RNAseq.
These data will provide us the transcriptional events associated specifically with progenitor mobilization and remyelination, and as such should provide new targets for accelerating and potentiating this process following acute demyelinating episodes.
New medium spiny neurons integrating into the brain’s existing striatopallidal circuitry.
Besides my group’s efforts in glial progenitor cell biology and use, we have also a longstanding interest in adult neurogenesis and its potential induction for therapeutic purposes.
In the course of my group’s longstanding work on adult neurogenesis in the songbird brain – a finding that I made as a graduate student many years ago and continue to investigate to the present day – we learned that two factors, the neurotrophic differentiation agent BDNF and the gliogenic suppressor noggin, are critical to permitting ongoing neuronal addition to adult brain tissue.
On that basis, we asked whether the forced over-expression of these agents in the forebrain subependyma, the region that harbors persistent neural stem cells, would be sufficient to direct neuronal addition to otherwise non-neurogenic regions of the adult mammalian brain. We found that this was indeed the case, and in a series of papers over the past decade, established BDNF and noggin over-expression as a feasible strategy by which to induce neuronal addition to the adult murine neostriatum.
Huntington's disease is a neurodegenerative disease characterized in part by the loss of neostriatal neurons, and in particularly of medium spiny striatopallidal projection neurons, the same phenotype recruited in response to BDNF and noggin. On that basis, we found that intracerebroventricular injection of adenoviral BDNF and noggin is indeed sufficient to not only trigger the addition of new neurons to the neostriatum, but also to slow disease progression in R6/2 mutant huntingtin mice.
More recently, we used intraventricular delivery of adeno-associated virus (AAV4), an ependymotrophic vector that allows persistent transgene expression, to sustain both BDNF and noggin expression and the recruitment of new medium spiny neurons in both wild-type and R6/2 mice. Using R6/2 x nestin-CreERT2 x EYFP mice treated with either intra-ventricular AAV4-BDNF/noggin, we proved the origin of these new cells from nestin+ subependymal progenitors, while pallidal backfills followed by patch clamp confirmed the maturation and circuit integration of these new neurons.
Most importantly, the AAV4-BDNF/noggin-treated R6/2 mice exhibited delayed motor deterioration and substantially increased survival. To validate the potential relevance of these observations to humans, we gave ICV injections of adenoviral BDNF and noggin to normal squirrel monkeys, in which we similarly observed significant treatment-associated striatal neuronal addition.
These observations suggest the potential feasibility of induced neurogenesis as a promising means of disease modification in HD. Going forward, they suggest the strong need and likely benefit of identifying necessary and sufficient signals for inducing neuronal differentiation from resident progenitor cells, and for regulating their adult parenchymal migration and circuit integration.
In addition, these observations suggest the need for new genetic targets and gene therapeutic strategies for inducing and supporting neuronal addition to the brain from resident progenitor cells. We have initiated these studies by profiling human medium spiny neuron progenitors, and by focusing on the receptors expressed by these cells, have predicted the ligands by which they may be supported and their differentiation influenced.
Using this information, we hope to design an increasingly more efficient and targeted strategy for the robust induction of neural addition to diseased or injured neural circuits throughout the brain, and not just those of the neostriatum. This rational search for new initiators and regulators of adult neuronal addition to extrastriatal brain regions will be focus of effort for years to come, as will be the optimization of adult neuronal addition strategies in those regions for which we already have effective induction protocols.
Astrocytes derived from an iPSC model of schizophrenia.
Glial form and function are extraordinarily divergent with evolution, and human astrocytes are virtually unique in their pleomorphism and fiber complexity. In particular, human astrocytes are larger and more structurally complex than rodent glia, and coordinate the actions of vastly more synapses within their geographic domains.
To assess the relative contributions of glial cells to the species-specific aspects of human cognition, we had previously engrafted neonatal mice with human glial progenitor cells (GPCs), to establish brains chimeric for human astrocytes. In collaboration with Dr. Nedergaard’s lab, we then assessed these glial chimeric mice functionally and behaviorally, and found that they exhibited substantially enhanced activity-dependent plasticity and learning.
This work established the potential of using human glial chimeras to assess human-specific aspects of the contributions of astrocytes to cognition, and to its disorders.
Such astrocytic involvement in human cognitive disorders has never been studied, and yet its role may be profound. As a case in point, a number of conditions, especially several neuropsychiatric disorders, are specific to humans. Yet while human neuronal cytoarchitecture is not very different from that of primates, astrocytic pleomorphism exhibits a quantal leap with human evolution, concurrent with the appearance of a number of neuropsychiatric conditions that appear unique to humans.
In particular, given the correlation between the appearance in humans of neuropsychiatric disorders such as schizophrenia and bipolar disorder, with that of uniquely human astrocytic morphologies and synaptic modulation, we are pursuing the specific possibility that human glial pathology contributes to the pathogenesis of schizophrenia.
To test this hypothesis, we have established human glial chimeric mice with glial progenitor cells derived from iPS cells themselves derived from patients with juvenile-onset schizophrenia, a category typically associated with a set of described mutations and copy number variants.
By this means, we have generated human glial chimeric mice whose glia have been largely replaced by human glial progenitors and their derived astrocytes, in which the selective effects of schizophrenia-derived glia, on both cortical neuroanatomy and behavior may be assessed, relative to those derived from normal patient controls.
This is a collaborative project with Maiken Nedergaard’s lab, which is responsible for the physiological and behavioral assessment of these animals, after the generation of the subject cells and chimeric animals by our group, which is also responsible for assessing the neuroanatomical and transcriptional concomitants of schizophrenia-derived human glial chimerization.
Our hope is that we may define new glial targets for the treatment of schizophrenia, a disease whose treatment strategies have hitherto largely been limited to the suppression of dopaminergic signaling.
A section of Human glial progenitor cell-implanted shiverer mouse brain, immunolabeled for MBP.
In the most clinically-advanced of the lab’s lines of investigation, we have established protocols for the identification, isolation and transplantation of human oligodendrocyte progenitor cells, which have succeeded in completely remyelinating the nervous systems of congenitally-hypomyelinated animals and rescuing normal neurological phenotype.
On the basis of this work, we have initiated a planned 4-year clinical trial of human OPC transplantation into patients with chronic progressive multiple sclerosis; this is a 4-year consortium effort spanning three medical schools in upstate New York (USA), and comprises the first attempt in adult humans of oligodendrocyte progenitor cell transplantation.
Human vs Rodent astrocytes. (Courtesy Alexi Verkhratsky (Chapter 3), Neuroglia by Kettenmann).
One of the most basic but fundamental questions underlying the study of brain science is understanding what factors account for the differences in interspecies intelligence and computational power.
Sophisticated cognitive abilities, such as an unparalleled capacity for learning, language, abstract expression, and metacognition are said to set humans apart from animals. But what accounts for these differences? Classically, interspecies variation in intelligence has been attributed to neurons, the electrically excitable subunit of all nervous systems.
However comparisons between the brains of different species indicate that the proportional make up and sophistication of a different, historically underappreciated, type of brain cell increases as a function of increased cognitive capability.
In species with more capable brains, astrocytes – a type of non-electrically excitable brain cell, also known as neuroglia— account for a considerably higher proportion of the brain.
More importantly, human astrocytes are 20-fold larger and much more complex than rodent astrocytes. The correlation between proportional makeup and intelligence suggests that astrocytes, which were first discovered by Rudolf Virchow and later Camillo Golgi, in the late 1800’s, may be more involved in sophisticated neural processes than previously considered.
Indeed, rapid advances in the field of glial biology have depicted a much more active role for astrocytes, one which is now widely considered to be critical for coordinating many basic physiological and higher order cognitive processes, such as control of respiratory rate and the dynamics of cerebral blood flow, regulation of the sleep wake cycle, and the facilitation of learning and memory, among others. Still remaining to be discovered are how human astrocytes functionally differ from their rodent counterparts and how these differences contribute to more complex information processing.
Mouse vs. human astrocytes
Nevertheless, observations of neuro-glial actions have spurred great interest in developing glial targeted treatments for neurological disease. Although a number of glial targeted therapies, such as the treatment of neuroinflammation and chronic pain, have been shown to be effective on animal models in the laboratory setting, they have largely failed to predict human outcomes.
The exact source of this discrepancy remains a contentious issue in science; however, recent research suggests that part of the reason may be due to differences in how astrocytes evolved in different species.
Efforts at contrasting the evolution of astrocytic form and function are ongoing, however, given that current neuroscience research and the development of treatments for neurological disease heavily depend on drawing parallels between humans and animal models, understanding the evolution and interspecies variability of astrocytes is particularly important.
Work in our lab has demonstrated that compared to rodents, the average protoplasmic astrocyte from the human neocortex exhibits 10-fold more primary processes and is more than 20-fold larger in volume. Interestingly, our analysis using two-photon imaging of the photolysis of caged Ca2+ in fine astrocytic projections revealed that the speed at which astrocytes communicate with each other, via the propagation of calcium waves, is also faster in humans than it is in rodents.
Neurofilament staining of human astrocytes
Our hope is that furthering our understanding of the evolution of astrocytes and how they differ between species will help overcome the obstacle of how to extrapolate and apply the findings from animal studies to human disease. Current work is focused on characterizing a fundamentally new way to study human glial cell interactions.
We recently generated chimeric mice that have been engrafted with human glial progenitor cells that differentiated into human astrocytes. In an unexpected finding, as the engrafted human cells developed, they displaced the native mouse glial cells. Interestingly, the chimeric mice also exhibited heightened functions compared to control animals, such as improved learning and memory. As the first of its kind, this new model offers the unique opportunity to study the functions of human glial cells in animal models of neuropathologies, in vivo, in real time.
Our hope is this work will further our understanding of the differences between rodent and human glial cells, and that it may lead to new insights into why animal models of disease often fail to predict human outcomes.
This project is a collaboration between the Goldman Lab and the Nedergaard Lab.
Further Reading
Forebrain Engraftment by Human Glial Progenitor Cells Enhances Synaptic Plasticity and Learning in Adult Mice. Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, Silva AJ, Takano T, Goldman SA, Nedergaard M. Cell Stem Cell. 2013 Mar 7;12, 342–353.
Heterogeneity of Astrocytic Form and Function. Oberheim NA, Goldman SA, Nedergaard M. Methods Mol Biol. 2012;814:23-45. doi: 10.1007/978-1-61779-452-0_3. Review. PMID: 22144298.
Uniquely hominid Features of Adult Human Astrocytes. Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, Ransom BR, Goldman SA, Nedergaard M. J Neurosci. 2009 Mar 11;29(10):3276-87. doi: 10.1523/JNEUROSCI.4707-08.2009. PMID: 19279265.
Astrocytic Complexity Distinguishes the Human Brain. Oberheim NA, Wang X, Goldman S, Nedergaard M. Trends Neurosci. 2006 Oct;29(10):547-53. Epub 2006 Aug 30. PMID: 16938356

